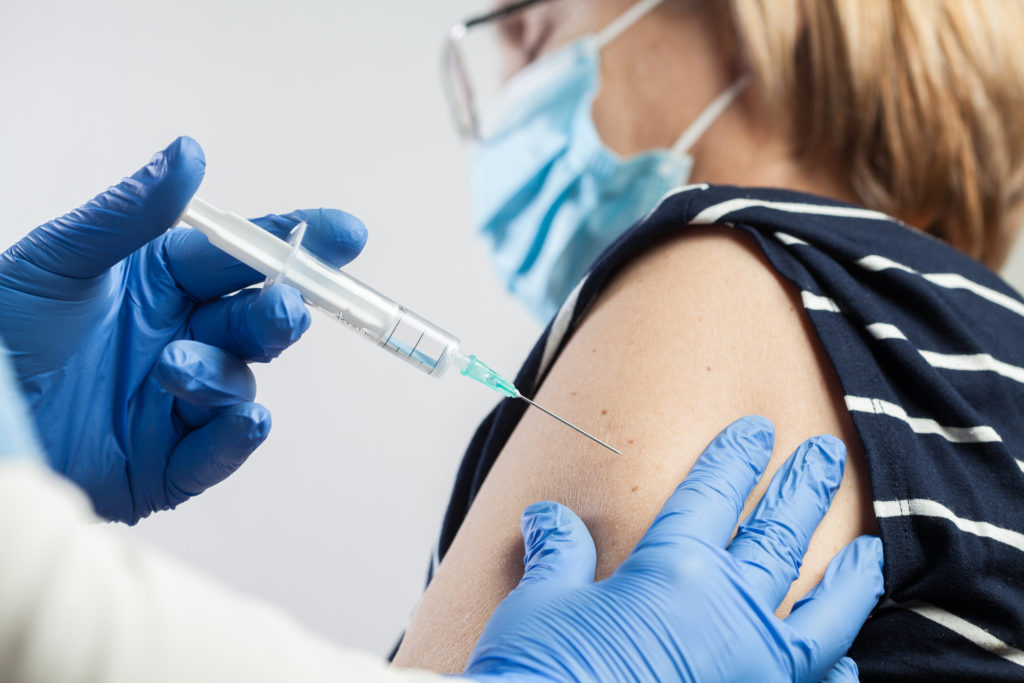
Pfizer Inc (NYSE: PFE) said on Monday its COVID-19 vaccine developed in collaboration with Germany’s BioNTech is just as effective in children (5- to-11-year-olds) as it was in adults. The Phase II and III clinical trials also established the vaccine as generally safe for children.
The U.S. pharmaceutical giant now plans on requesting authorisation from the United States and European regulators “as soon as possibleâ€.
Dr Gottlieb’s remarks on CNBC’s “Squawk Boxâ€
Are you looking for fast-news, hot-tips and market analysis?
Sign-up for the Invezz newsletter, today.
According to the former FDA commissioner, Dr Scott Gottlieb, Pfizer’s COVID-19 shot could be available for children by the end of October. In his interview with CNBC’s “Squawk Box†this morning, he said:
It’s a 10-microgram dose, so it’s effectively the same vaccine that’s available for adults over the age of 12, just in a lower dose. The antibody response was also comparable with this lower dose to what you saw in people aged 16 to 25.
Pfizer intends to use different sized vials for the 10 mg dose to minimise the risk of contamination, but the need for new vials was unlikely to slow down things, Dr Gottlieb confirmed.
Pfizer focused on making its vaccine more tolerable for children
On top of efficacy, Dr Gottlieb added, Pfizer also focused on making its shot more tolerable for children to minimise vaccine-related side effects like fever and injection site reactions. The American multinational tested three different doses with the 10 mg dose “striking the best balance between efficacy and tolerabilityâ€.
Pediatric cases of the COVID-19 in the United States have climbed by roughly 240% since July. The sharp increase, as per Pfizer’s CEO Albert Bourla, “underscored the public health need for vaccination in childrenâ€.
Pfizer shares are about 1.0% up on Monday. The $246 billion company now has a price to earnings ratio of 18.77.
eToro
10/10
67% of retail CFD accounts lose money